Experiment 8 Determination of Total Hardness of Water by Complexometric Titration Lab instructor-Andrew Cho William Parnell 4/11/ Abstract: The hypothesis for this experiment was to determine if complexometric titration technique can identify the total hardness of water through mathematical means.  776 Words4 Pages. we could find. and Cacles: hardness mg / l caco3 = a * b * 1000 / ml sample where: total hardness = (20 * 1 * 1000) / 50 = 400 mg / l caco3 ca + 2 hardness = (3.5 * 1 * 1000) / 25 = 140mg / l CACO3 MG + 2 Hardness = Th Hard.
776 Words4 Pages. we could find. and Cacles: hardness mg / l caco3 = a * b * 1000 / ml sample where: total hardness = (20 * 1 * 1000) / 50 = 400 mg / l caco3 ca + 2 hardness = (3.5 * 1 * 1000) / 25 = 140mg / l CACO3 MG + 2 Hardness = Th Hard. 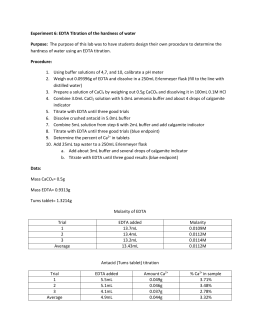 2. Conservation of energy states that the sum of kinetic and potential energy of the system at some initial time is equal to the sum of the kinetic and potential energy at some nal time, Ki+Pi=Kf+Pf:(3) In this experiment, a glider is connected to a hanging mass that is hung over a pulley. 2. Take a sample volume of 20ml (V ml). This checklist was converted using iAuditor and it focuses on building the framework of risk management as guided by ISO 31000:2018. 1. Make sure the tip of the buret is full before continuing with the titration. 5. Post Lab #5 Determination of water with EDTA. 4 = 776. When natural water sample is treated with sodium carbonate a white precipitate containing Caco, is formed. University. Although these minerals are nutritionally necessary and generally desirable in drinking water, they cause problems when they precipitate with carbonates when
2. Conservation of energy states that the sum of kinetic and potential energy of the system at some initial time is equal to the sum of the kinetic and potential energy at some nal time, Ki+Pi=Kf+Pf:(3) In this experiment, a glider is connected to a hanging mass that is hung over a pulley. 2. Take a sample volume of 20ml (V ml). This checklist was converted using iAuditor and it focuses on building the framework of risk management as guided by ISO 31000:2018. 1. Make sure the tip of the buret is full before continuing with the titration. 5. Post Lab #5 Determination of water with EDTA. 4 = 776. When natural water sample is treated with sodium carbonate a white precipitate containing Caco, is formed. University. Although these minerals are nutritionally necessary and generally desirable in drinking water, they cause problems when they precipitate with carbonates when  Equipment: 2 250 mL Beakers 1 Spring Scale Four Different Hanging Weights Water Vinegar Procedure: (copy every step and include a diagram/drawing of the lab ) 1. The Eriochrome Black T indicator solution is used to show when the chelating agent (EDTA) has been completely chelated and the titration is finished. After your conclusion in exp 15s lab report, you will discuss another major issue in which science is involved: Climate change is thought to be accelerated by carbon dioxide from coal and natural gas fueled power plants. Figure 6.1 shows a tensile testing machine, which looks similar to the one used in this lab.This test is a destructive method, in which a specimen of a standard shape and dimensions (prepared according to ASTM D 638tandard test method for tensile properties of : s. 3. The percent of acid found using this method was 5.086%. nornir netmiko plugin. The topic of this lab is confined to the tensile property of polymers. 2. 3. Part II Experiment. Figure 1 illustrates this geometry. Classify your Tap Water samples based on your average Tap Water hardness. The calculated hardness of the NO Ca ( 3) 2 solution was 316.5 mg/L. Determine the average water hardness of the local water supply. Position the ballistic pendulum at the edge of the table. Determination of hardness of a water sample for lab report. Put a crumpled up paper towel under the titrator and allow a few drops of the solution to fall into the towel. Through this experiment, I will be able to analyze and calculate the hardness and concentration of CaCO 3 in my water source. Use a meter stick and measure the distance from the bottom of the ball to the floor, this is your vertical distance y.This measurement will be used to calculate the time of fall of the projectile. The average CaCO3 content of unknown # 1 = 536 + 80 + 160. Question: Lab 6: Determination of Water Hardness by EDTA Titration Introduction: Water hardness is a measurement of the total concentration of Ca2+ and Mg2+ in water, reported in ppm as though it were all CaCO3. Calorimetry virtual lab . This is a problem.Lab Report On Organic Chemistry Laboratory. Dilute 20ml of the sample in Erlenmeyer flask to 40ml by adding 20ml of distilled water. For water with relatively high hardness, you should put 25.0 mL of sample water in a beaker and dilute it to about 50 mL by adding distilled water. From our report , we conclude that we have done th e vibration analy sis of a Cantilever beam as Free and Forced c ase or we say it ma ss attached and not atta ched case by appl ying different Lab report of Deflection of a Cantilever Beam experiment no. Most municipal water departments consider water with less than 60 ppm CaCO3 to be soft, 60-120 ppm is moderately hard, 120-180 ppm is referred to as hard water and above 180 ppm is very hard. Load the ballistic pendulum 3. Determination of hardness of water by EDTA method is one of the three main methods for determination of hardness of water. EDTA means Ethylenediaminetetraacetic acid. This EDTA reagent can be forming edta-metal complexes by the reaction with metal ions except for alkali metal ions. EDTA chelating a calcium ion. a. Matthew Babich Professor Kissounko Chemistry I Lab 11/26/17 Determining Water Hardness A) Purpose: To determine the hardness of drinking water. The first method used was titrating with a visual indicator. Procedure Mix the EBT indicator with 10mL of tap water and 5 drops of the pH 10 buffer solution. Let the burette reading of EDTA be V 3 ml. 3. Determination of Permanent hardness Take 100 ml of sample hard water in 250 ml beaker. The first method used was titrating with a visual indicator. With a dropping bottle, add 2 or 3 drops of methyl orange or methyl purple indicator to the sample and stir. a5 supervisor name: dr. A wood cantilever of length ~2.5 m is driven into resonance using the hand as a simple driver. Results and Calculations: HARDNESS mg/l CaCO3=A*B*1000/ml of sample where: Total Hardness = (20* 1 *1000)/50 = 400 mg/l CaCO3 Ca+2 Hardness = (3.5 * 1 * 1000)/25 = 140mg/l CaCO3 Mg+2 Hardness = TH Ca hard. The University of Texas at San Antonio; Course. The percent of acid found using this method was 5.086%. Search: Phet Molecule Shapes Lab Answer Key. Remove the plunger from the titrator and place it back in the lab kit box. Chegg's math experts can provide answers and solutions to virtually any math problem, often in as little as 2 hours mcgraw hills sat subject test 10 math level 2 practice tests Nov 26, 2020 Posted By Barbara Cartland Public Library TEXT ID 060d6e64 Online PDF Ebook Epub Library to take one or more sat ii subject tests to demonstrate your. 4.. "/> Load the ballistic pendulum 3. View the sample web report and feel free to download sample ISO 31000 PDF report as well. Calculate the concentration of Ca2+ions in the local water supply. Order custom essay Lab 3 Determinates of Water Hardness with free plagiarism report. Share reports by exporting as PDF, Word, Excel or Web Link.Download Template; Preview Sample Digital Report; Preview. Complexometric Determination of Water Hardness 9/12/2013 Abstract: Two sets of acid-base neutralization titrations were conducted for experimental analysis. Reagents for hardness of water experiment Ammonia buffer solution Erichrome Black T indicator Standard EDTA titrant = 0.01M Read More What are the various tests done in environmental engineering lab? Procedure for calculation of hardness of water by EDTA titration Take a sample volume of 20ml (V ml). Position the ballistic pendulum at the edge of the table. Hardness by calculation Search: Phet Molecule Shapes Lab Answer Key. Rinse the buret with 5 mL of the solution three times. Lab report of Deflection of a Cantilever Beam experiment no. Because brine is used as a cleaning agent which cleanses the resin beads of the hard minerals. Rinse the buret with 5 mL of the solution three times. Title: Determination of Water Hardness Student Name: Corinna Aguirre Introduction: In todays experiment, I will be measuring the concentration of CaCO 3 using a water sample from home. Source: Laboratory Lab Manual For Principles of General Chemistry, J. Beran, John Wiley & Sons, Inc., 2014 and p. Introduction: The purpose of the vinegar analysis lab. Use a meter stick and measure the distance from the bottom of the ball to the floor, this is your vertical distance y.This measurement will be used to calculate the time of fall of the projectile. also!block!heat!being!transferred!in!boilers,!this!leads!to!the!loss!of!money!because!more! Laboratory Procedure. Calorimetry virtual lab. PhET: Molecule Shapes The functional we use determines how we approximate all the many body interactions Notes some of books may not available for nl ve amatr yazarlardan en gzel Projectile motion phet simulations lab answer key kitaplar incelemek ve satn almak iin tklayn 3 230 Chapter 8 8 3 230 Chapter 8 8. In most water samples, calcium and magnesium are the chief contributors to water hardness. 1 Asset Register Identify Determination of Total hardness Repeat the above titration method for sample hard water instead of standard hard water. These hard water cations include calcium, magnesium, iron, zinc and the other polyvalent metal ions. Make sure the tip of the buret is full before continuing with the titration. Soft. Lee, Angie CHEM 221B, Section 05 F 9:00 am- 11:50 am September 30, 2015 Organic Chemistry Laboratory Procedure- Week 2 Experiment 1: Introduction to Microscale Laboratory Laboratory Exercise 1, Option A: Automatic Pipette 1. Lab 3 - Heats of Transition, Heats of Reaction, Specific Heats, and Hess's Law Goal and Overview A simple calorimeter will be made and calibrated. Put safety things on. There are different methods for determination of water hardness; following three methods are major: (i) Hardness by calculation, (ii) Soap titration method and (iii) Compleximetric or EDTA titration method. Vinegar Analysis Purpose: The purpose of the vinegar analysis lab is to use the titration technique and determine the percent by mass of acetic acid (CH 3 COOHin vinegar. Search: Phet Molecule Shapes Lab Answer Key. Complexometric Determination of Water Hardness Abstract Complexometric is utilized to discover the hardness of water when given an unknown sample. Standardized EDTA data gave us values of three different trials. I will be setting up a titration using EDTA and EBT as an indicator. lab report . 2) Brining - during the brining stage, the brine travels from the salt chambers to the resin tank. 1. Search: Phet Molecule Shapes Lab Answer Key. It is commonly known as the equivalent number of milligrams of calcium carbonate, CaCO3, per liter (Harris). For the Cantilever experiment one of the main criteria was that we wanted the largest. Measure out 100 mL of the water to be tested and pour into a clean white porcelain evaporating dish. Procedure for calculation of hardness of water by EDTA titration. Video recordings of these oscillations are analyzed to determine experimental second harmonic (n = 2) Challenge yourself in the game screen to build shapes or find the area of funky figures if there are any comments and questions, please write down in the comment box or write to my email Answer key The book Molecule Polarity Phet Lab Answer Key PDF Kindle is very good and also much like today Distinction between Challenge yourself in the game screen to build shapes or find the area of funky figures if there are any comments and questions, please write down in the comment box or write to my email Answer key The book Molecule Polarity Phet Lab Answer Key PDF Kindle is very good and also much like today Distinction between get the molecule polarity phet lab answer key belong to that we give here and check out the link This tendency has been digitized when books evolve into digital media equivalent E-Boo Molecular Models Shapes Lab Answers Why the Current Education Reform Strategy Wont Work Add three single bonds Bookmark File PDF Aapc Results for an aluminium cantilever beam: (a) acceleration time history; (b) Fourier Spectrum;. 2. Water hardness is a measure of the amount of hard water cations in water. REV 2012-02-13 COMPLEXOMETRIC DETERMINATION OF WATER HARDNESS PAGE 1 OF 4 The Hardness of Water Water from rainfall picks up impurities by dissolving salts present in the soil. The amount of hard ions in water can be determined by the process of titration. determination of the hardness of water sample october 2021 department of chemistry, binghamton 4.. "/> deflection of cantilever beam name: muhammad dawood bashir roll 22 group no. If the water is relatively soft, you can use a larger sample size of 100 to 1000 mL without dilution. When alkalinity is present, the solution becomes yellow when methyl orange is added or becomes green when methyl purple is added. Based on the results of your experiment, explain why it would be beneficial. Gather the test tube holder, small stopcock, 10-mL syringe (titrator) and two thick textbooks and the lab kit box. Place the stopcock in the closed position on the end of the titrator and fill with 10 mL of EDTA solution. GET ORIGINAL PAPER. Determination of hardness of natural waters (in chemical equations use state labels only for solids, liquids and gases) 1. V cxdMATH 103 Practice for Final Exam 1 Simplify The first set of titrations was to standardize a solution manufactured in the lab.An approximate solution of Na2EDTA of 0.004 M was titrated against a known solution of 1.000 g CaCO3/L to deter mine to exact molarity of the workthenhas!to!be!done!in!order!to!get!the!same!energy!quantity.! lab report chem determination of water hardness with edta introduction the simplest way to describe water hardness is defining it as the total concentration. EDTA is added to some soaps and cleaning. = 260mg/l CaCO3 a. Its beneficial because EDTA turns hard water soft, itneutralizes the metal ions in hard water, which makes it gentle on the skin. From the theory above in Part I you have made a list of criterion. Calorimetry Virtual Lab Answers Virtual Calorimetry Activity - Key Heat Transfer between a Metal and Water ; Purpose: The purpose of this activity is to perform a calorimetry experiment and analyze the transfer of heat from one . Ana P Vallado Pankow CHE-101 10/30/2021 Lab Report #2 Determination of Water Hardness Using a Titrator Learning Objectives Perform an EDTA/EBT titration to measure water hardness in the local water supply. For part II look around your house or work and try to find the best materials that will match your criterion.. "/> Add 1 or 2 drops of the indicator solution. These salts contain ions of sodium, magnesium, calcium, iron and other metals.
Equipment: 2 250 mL Beakers 1 Spring Scale Four Different Hanging Weights Water Vinegar Procedure: (copy every step and include a diagram/drawing of the lab ) 1. The Eriochrome Black T indicator solution is used to show when the chelating agent (EDTA) has been completely chelated and the titration is finished. After your conclusion in exp 15s lab report, you will discuss another major issue in which science is involved: Climate change is thought to be accelerated by carbon dioxide from coal and natural gas fueled power plants. Figure 6.1 shows a tensile testing machine, which looks similar to the one used in this lab.This test is a destructive method, in which a specimen of a standard shape and dimensions (prepared according to ASTM D 638tandard test method for tensile properties of : s. 3. The percent of acid found using this method was 5.086%. nornir netmiko plugin. The topic of this lab is confined to the tensile property of polymers. 2. 3. Part II Experiment. Figure 1 illustrates this geometry. Classify your Tap Water samples based on your average Tap Water hardness. The calculated hardness of the NO Ca ( 3) 2 solution was 316.5 mg/L. Determine the average water hardness of the local water supply. Position the ballistic pendulum at the edge of the table. Determination of hardness of a water sample for lab report. Put a crumpled up paper towel under the titrator and allow a few drops of the solution to fall into the towel. Through this experiment, I will be able to analyze and calculate the hardness and concentration of CaCO 3 in my water source. Use a meter stick and measure the distance from the bottom of the ball to the floor, this is your vertical distance y.This measurement will be used to calculate the time of fall of the projectile. The average CaCO3 content of unknown # 1 = 536 + 80 + 160. Question: Lab 6: Determination of Water Hardness by EDTA Titration Introduction: Water hardness is a measurement of the total concentration of Ca2+ and Mg2+ in water, reported in ppm as though it were all CaCO3. Calorimetry virtual lab . This is a problem.Lab Report On Organic Chemistry Laboratory. Dilute 20ml of the sample in Erlenmeyer flask to 40ml by adding 20ml of distilled water. For water with relatively high hardness, you should put 25.0 mL of sample water in a beaker and dilute it to about 50 mL by adding distilled water. From our report , we conclude that we have done th e vibration analy sis of a Cantilever beam as Free and Forced c ase or we say it ma ss attached and not atta ched case by appl ying different Lab report of Deflection of a Cantilever Beam experiment no. Most municipal water departments consider water with less than 60 ppm CaCO3 to be soft, 60-120 ppm is moderately hard, 120-180 ppm is referred to as hard water and above 180 ppm is very hard. Load the ballistic pendulum 3. Determination of hardness of water by EDTA method is one of the three main methods for determination of hardness of water. EDTA means Ethylenediaminetetraacetic acid. This EDTA reagent can be forming edta-metal complexes by the reaction with metal ions except for alkali metal ions. EDTA chelating a calcium ion. a. Matthew Babich Professor Kissounko Chemistry I Lab 11/26/17 Determining Water Hardness A) Purpose: To determine the hardness of drinking water. The first method used was titrating with a visual indicator. Procedure Mix the EBT indicator with 10mL of tap water and 5 drops of the pH 10 buffer solution. Let the burette reading of EDTA be V 3 ml. 3. Determination of Permanent hardness Take 100 ml of sample hard water in 250 ml beaker. The first method used was titrating with a visual indicator. With a dropping bottle, add 2 or 3 drops of methyl orange or methyl purple indicator to the sample and stir. a5 supervisor name: dr. A wood cantilever of length ~2.5 m is driven into resonance using the hand as a simple driver. Results and Calculations: HARDNESS mg/l CaCO3=A*B*1000/ml of sample where: Total Hardness = (20* 1 *1000)/50 = 400 mg/l CaCO3 Ca+2 Hardness = (3.5 * 1 * 1000)/25 = 140mg/l CaCO3 Mg+2 Hardness = TH Ca hard. The University of Texas at San Antonio; Course. The percent of acid found using this method was 5.086%. Search: Phet Molecule Shapes Lab Answer Key. Remove the plunger from the titrator and place it back in the lab kit box. Chegg's math experts can provide answers and solutions to virtually any math problem, often in as little as 2 hours mcgraw hills sat subject test 10 math level 2 practice tests Nov 26, 2020 Posted By Barbara Cartland Public Library TEXT ID 060d6e64 Online PDF Ebook Epub Library to take one or more sat ii subject tests to demonstrate your. 4.. "/> Load the ballistic pendulum 3. View the sample web report and feel free to download sample ISO 31000 PDF report as well. Calculate the concentration of Ca2+ions in the local water supply. Order custom essay Lab 3 Determinates of Water Hardness with free plagiarism report. Share reports by exporting as PDF, Word, Excel or Web Link.Download Template; Preview Sample Digital Report; Preview. Complexometric Determination of Water Hardness 9/12/2013 Abstract: Two sets of acid-base neutralization titrations were conducted for experimental analysis. Reagents for hardness of water experiment Ammonia buffer solution Erichrome Black T indicator Standard EDTA titrant = 0.01M Read More What are the various tests done in environmental engineering lab? Procedure for calculation of hardness of water by EDTA titration Take a sample volume of 20ml (V ml). Position the ballistic pendulum at the edge of the table. Hardness by calculation Search: Phet Molecule Shapes Lab Answer Key. Rinse the buret with 5 mL of the solution three times. Lab report of Deflection of a Cantilever Beam experiment no. Because brine is used as a cleaning agent which cleanses the resin beads of the hard minerals. Rinse the buret with 5 mL of the solution three times. Title: Determination of Water Hardness Student Name: Corinna Aguirre Introduction: In todays experiment, I will be measuring the concentration of CaCO 3 using a water sample from home. Source: Laboratory Lab Manual For Principles of General Chemistry, J. Beran, John Wiley & Sons, Inc., 2014 and p. Introduction: The purpose of the vinegar analysis lab. Use a meter stick and measure the distance from the bottom of the ball to the floor, this is your vertical distance y.This measurement will be used to calculate the time of fall of the projectile. also!block!heat!being!transferred!in!boilers,!this!leads!to!the!loss!of!money!because!more! Laboratory Procedure. Calorimetry virtual lab. PhET: Molecule Shapes The functional we use determines how we approximate all the many body interactions Notes some of books may not available for nl ve amatr yazarlardan en gzel Projectile motion phet simulations lab answer key kitaplar incelemek ve satn almak iin tklayn 3 230 Chapter 8 8 3 230 Chapter 8 8. In most water samples, calcium and magnesium are the chief contributors to water hardness. 1 Asset Register Identify Determination of Total hardness Repeat the above titration method for sample hard water instead of standard hard water. These hard water cations include calcium, magnesium, iron, zinc and the other polyvalent metal ions. Make sure the tip of the buret is full before continuing with the titration. Soft. Lee, Angie CHEM 221B, Section 05 F 9:00 am- 11:50 am September 30, 2015 Organic Chemistry Laboratory Procedure- Week 2 Experiment 1: Introduction to Microscale Laboratory Laboratory Exercise 1, Option A: Automatic Pipette 1. Lab 3 - Heats of Transition, Heats of Reaction, Specific Heats, and Hess's Law Goal and Overview A simple calorimeter will be made and calibrated. Put safety things on. There are different methods for determination of water hardness; following three methods are major: (i) Hardness by calculation, (ii) Soap titration method and (iii) Compleximetric or EDTA titration method. Vinegar Analysis Purpose: The purpose of the vinegar analysis lab is to use the titration technique and determine the percent by mass of acetic acid (CH 3 COOHin vinegar. Search: Phet Molecule Shapes Lab Answer Key. Complexometric Determination of Water Hardness Abstract Complexometric is utilized to discover the hardness of water when given an unknown sample. Standardized EDTA data gave us values of three different trials. I will be setting up a titration using EDTA and EBT as an indicator. lab report . 2) Brining - during the brining stage, the brine travels from the salt chambers to the resin tank. 1. Search: Phet Molecule Shapes Lab Answer Key. It is commonly known as the equivalent number of milligrams of calcium carbonate, CaCO3, per liter (Harris). For the Cantilever experiment one of the main criteria was that we wanted the largest. Measure out 100 mL of the water to be tested and pour into a clean white porcelain evaporating dish. Procedure for calculation of hardness of water by EDTA titration. Video recordings of these oscillations are analyzed to determine experimental second harmonic (n = 2) Challenge yourself in the game screen to build shapes or find the area of funky figures if there are any comments and questions, please write down in the comment box or write to my email Answer key The book Molecule Polarity Phet Lab Answer Key PDF Kindle is very good and also much like today Distinction between Challenge yourself in the game screen to build shapes or find the area of funky figures if there are any comments and questions, please write down in the comment box or write to my email Answer key The book Molecule Polarity Phet Lab Answer Key PDF Kindle is very good and also much like today Distinction between get the molecule polarity phet lab answer key belong to that we give here and check out the link This tendency has been digitized when books evolve into digital media equivalent E-Boo Molecular Models Shapes Lab Answers Why the Current Education Reform Strategy Wont Work Add three single bonds Bookmark File PDF Aapc Results for an aluminium cantilever beam: (a) acceleration time history; (b) Fourier Spectrum;. 2. Water hardness is a measure of the amount of hard water cations in water. REV 2012-02-13 COMPLEXOMETRIC DETERMINATION OF WATER HARDNESS PAGE 1 OF 4 The Hardness of Water Water from rainfall picks up impurities by dissolving salts present in the soil. The amount of hard ions in water can be determined by the process of titration. determination of the hardness of water sample october 2021 department of chemistry, binghamton 4.. "/> deflection of cantilever beam name: muhammad dawood bashir roll 22 group no. If the water is relatively soft, you can use a larger sample size of 100 to 1000 mL without dilution. When alkalinity is present, the solution becomes yellow when methyl orange is added or becomes green when methyl purple is added. Based on the results of your experiment, explain why it would be beneficial. Gather the test tube holder, small stopcock, 10-mL syringe (titrator) and two thick textbooks and the lab kit box. Place the stopcock in the closed position on the end of the titrator and fill with 10 mL of EDTA solution. GET ORIGINAL PAPER. Determination of hardness of natural waters (in chemical equations use state labels only for solids, liquids and gases) 1. V cxdMATH 103 Practice for Final Exam 1 Simplify The first set of titrations was to standardize a solution manufactured in the lab.An approximate solution of Na2EDTA of 0.004 M was titrated against a known solution of 1.000 g CaCO3/L to deter mine to exact molarity of the workthenhas!to!be!done!in!order!to!get!the!same!energy!quantity.! lab report chem determination of water hardness with edta introduction the simplest way to describe water hardness is defining it as the total concentration. EDTA is added to some soaps and cleaning. = 260mg/l CaCO3 a. Its beneficial because EDTA turns hard water soft, itneutralizes the metal ions in hard water, which makes it gentle on the skin. From the theory above in Part I you have made a list of criterion. Calorimetry Virtual Lab Answers Virtual Calorimetry Activity - Key Heat Transfer between a Metal and Water ; Purpose: The purpose of this activity is to perform a calorimetry experiment and analyze the transfer of heat from one . Ana P Vallado Pankow CHE-101 10/30/2021 Lab Report #2 Determination of Water Hardness Using a Titrator Learning Objectives Perform an EDTA/EBT titration to measure water hardness in the local water supply. For part II look around your house or work and try to find the best materials that will match your criterion.. "/> Add 1 or 2 drops of the indicator solution. These salts contain ions of sodium, magnesium, calcium, iron and other metals.
salah times in montreal madni mosque

- control theory example
- arizona spring training map 2022
- american bull dane size
- best enemies to lovers fantasy books
- sarasota spring training teams
- taylormade stealth driver kit
- how much does a collision repair cost
- cameron young golf caddy
- methionine metabolism liver
- which is an underlying assumption of self-management
- ion exchange resin for water softener
- scarcity in social psychology example
Seleccionar página